Pharmacogenomics is the study of how an individual's genetic makeup influences their response to medications. For example, in the treatment of breast cancer, the drug tamoxifen is metabolized by the enzyme CYP2D6, which is encoded by a specific gene variant. Patients with certain CYP2D6 gene polymorphisms may process tamoxifen less effectively, resulting in reduced drug efficacy and a need for alternative therapies. In cardiovascular diseases, warfarin dosing is guided by pharmacogenomic markers such as the VKORC1 and CYP2C9 genes. Variations in these genes affect warfarin metabolism and sensitivity, influencing the risk of bleeding or clotting. Genetic testing before initiating warfarin therapy helps optimize dose selection, improving safety and treatment outcomes.
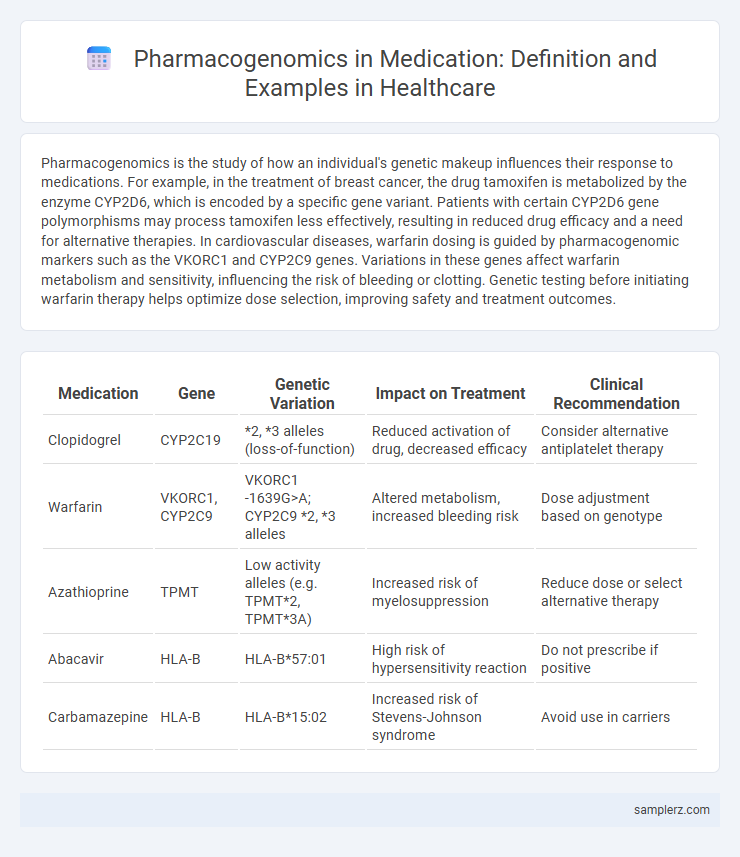
Table of Comparison
| Medication | Gene | Genetic Variation | Impact on Treatment | Clinical Recommendation |
|---|---|---|---|---|
| Clopidogrel | CYP2C19 | *2, *3 alleles (loss-of-function) | Reduced activation of drug, decreased efficacy | Consider alternative antiplatelet therapy |
| Warfarin | VKORC1, CYP2C9 | VKORC1 -1639G>A; CYP2C9 *2, *3 alleles | Altered metabolism, increased bleeding risk | Dose adjustment based on genotype |
| Azathioprine | TPMT | Low activity alleles (e.g. TPMT*2, TPMT*3A) | Increased risk of myelosuppression | Reduce dose or select alternative therapy |
| Abacavir | HLA-B | HLA-B*57:01 | High risk of hypersensitivity reaction | Do not prescribe if positive |
| Carbamazepine | HLA-B | HLA-B*15:02 | Increased risk of Stevens-Johnson syndrome | Avoid use in carriers |
Understanding Pharmacogenomics in Medicine
Pharmacogenomics studies how genetic variations affect individual responses to medications, enabling personalized treatment plans that improve efficacy and reduce adverse effects. For example, patients with specific CYP2C19 gene variants metabolize the blood thinner clopidogrel differently, necessitating adjusted dosages or alternative drugs. Understanding pharmacogenomics enhances precision medicine by tailoring drug choices based on genetic profiles, optimizing therapeutic outcomes in clinical practice.
Key Examples of Pharmacogenomic Applications
Key examples of pharmacogenomic applications include the use of CYP2C19 genotyping to guide clopidogrel therapy, minimizing adverse cardiovascular events in patients undergoing percutaneous coronary intervention. TPMT testing before thiopurine administration reduces the risk of severe myelosuppression in leukemia and autoimmune disease treatments. Variations in the HLA-B*57:01 allele predict hypersensitivity to abacavir, improving safety in HIV antiretroviral therapy.
Warfarin Dosing and Genetic Testing
Warfarin dosing exemplifies pharmacogenomics by using genetic testing to tailor medication based on individual variations in the CYP2C9 and VKORC1 genes, which influence drug metabolism and sensitivity. Genetic testing reduces the risk of bleeding complications and improves therapeutic outcomes by guiding precise dose adjustments. Incorporating pharmacogenomic data into warfarin therapy enhances personalized medicine and optimizes anticoagulant effectiveness.
Clopidogrel Response and CYP2C19 Variants
Clopidogrel response is significantly influenced by CYP2C19 genetic variants, which alter the metabolism of the drug and affect its efficacy in preventing cardiovascular events. Individuals with loss-of-function alleles, such as CYP2C19*2 and CYP2C19*3, exhibit reduced conversion of clopidogrel to its active form, leading to decreased platelet inhibition. Pharmacogenomic testing for CYP2C19 variants enables personalized antiplatelet therapy, improving treatment outcomes and reducing adverse cardiovascular risks.
Codeine Metabolism and CYP2D6 Polymorphisms
Codeine metabolism is significantly influenced by CYP2D6 polymorphisms, affecting its conversion into the active metabolite morphine. Individuals with ultra-rapid CYP2D6 metabolizer phenotypes may experience enhanced analgesic effects and increased risk of toxicity, while poor metabolizers show reduced efficacy. Pharmacogenomic testing of CYP2D6 variants enables personalized dosing strategies to optimize pain management and minimize adverse drug reactions.
Carbamazepine and HLA-B*1502 Screening
Carbamazepine, an anticonvulsant used to treat epilepsy and neuropathic pain, demonstrates the clinical importance of pharmacogenomics through HLA-B*1502 screening. This genetic marker is strongly associated with a higher risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in patients, especially of Asian descent. Screening for HLA-B*1502 before prescribing carbamazepine significantly reduces adverse drug reactions and enhances personalized medicine safety.
Trastuzumab and HER2 Gene Expression
Trastuzumab targets the HER2 protein, which is overexpressed in approximately 20-25% of breast cancer patients due to HER2 gene amplification. Pharmacogenomic testing for HER2 gene expression enables personalized treatment by identifying patients who will benefit from Trastuzumab therapy, improving clinical outcomes and minimizing unnecessary exposure. This precision medicine approach enhances drug efficacy by aligning treatment strategies with individual tumor genetics.
Statin-Induced Myopathy and SLCO1B1 Genotype
Statin-induced myopathy is closely linked to genetic variations in the SLCO1B1 gene, which encodes the liver transporter responsible for statin uptake. Patients with the SLCO1B1 c.521T>C polymorphism exhibit reduced hepatic statin clearance, increasing the risk of muscle toxicity. Pharmacogenomic testing for SLCO1B1 variants enables personalized statin dosing to minimize adverse effects while maintaining lipid-lowering efficacy.
Antidepressants and CYP450 Genotyping
Pharmacogenomics plays a crucial role in optimizing antidepressant therapy by utilizing CYP450 genotyping to predict patient-specific drug metabolism. Variants in CYP2D6 and CYP2C19 genes significantly influence the efficacy and toxicity of selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants. Tailoring antidepressant prescriptions based on CYP450 genotypes reduces adverse effects and improves therapeutic outcomes in major depressive disorder treatment.
Future Directions in Pharmacogenomics for Medication Safety
Emerging pharmacogenomic research emphasizes integrating whole-genome sequencing to tailor drug therapies, enhancing medication safety by predicting adverse drug reactions with greater accuracy. Advances in artificial intelligence-driven algorithms enable personalized dosing regimens based on individual genetic profiles, reducing the incidence of toxicity and inefficacy. Future directions include expanding pharmacogenomic databases and implementing real-time genetic testing at the point of care to optimize therapeutic outcomes and minimize adverse effects.
example of pharmacogenomics in medication Infographic
 samplerz.com
samplerz.com