Photodegradation in the atmosphere involves the breakdown of pollutants through the action of sunlight. One prominent example is the photodegradation of nitrogen dioxide (NO2), a common air pollutant produced by vehicle emissions and industrial processes. Exposure to ultraviolet (UV) light causes NO2 molecules to absorb energy and dissociate into nitric oxide (NO) and atomic oxygen (O), which then reacts with oxygen to form ozone (O3). Another example is the photodegradation of volatile organic compounds (VOCs), which contribute to smog formation. When VOCs encounter UV radiation, they undergo complex chemical reactions leading to the formation of secondary pollutants such as formaldehyde and peroxyacetyl nitrates (PANs). These processes significantly impact air quality and play a critical role in atmospheric chemistry and environmental health.
Table of Comparison
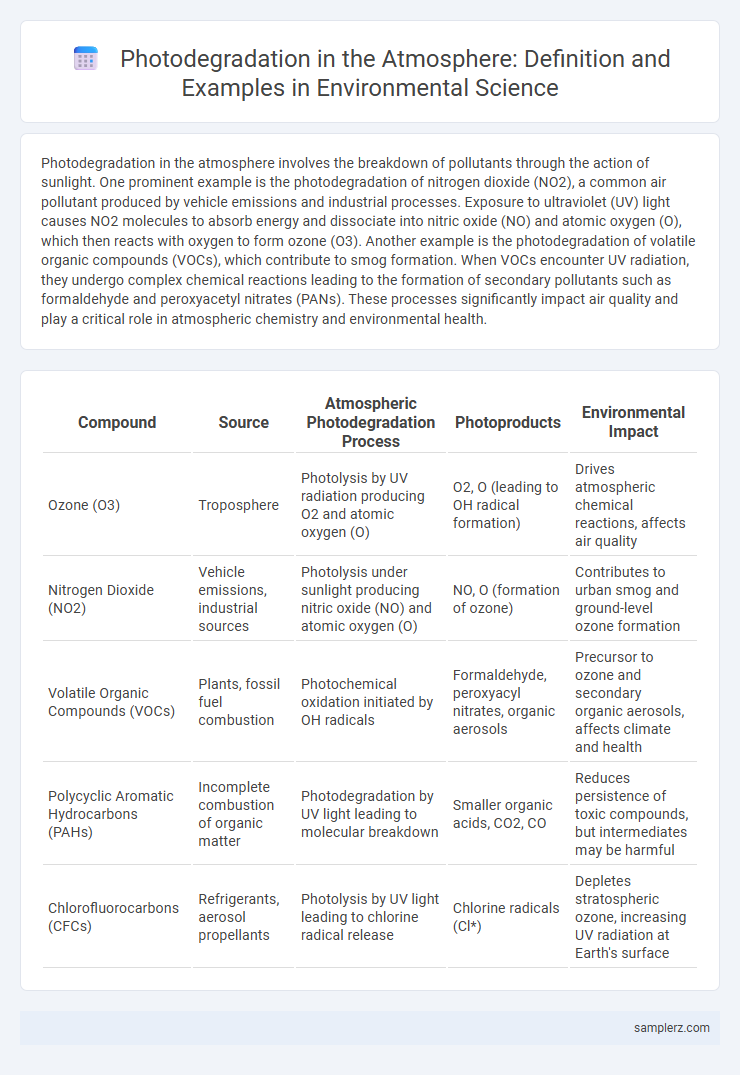
| Compound | Source | Atmospheric Photodegradation Process | Photoproducts | Environmental Impact |
|---|---|---|---|---|
| Ozone (O3) | Troposphere | Photolysis by UV radiation producing O2 and atomic oxygen (O) | O2, O (leading to OH radical formation) | Drives atmospheric chemical reactions, affects air quality |
| Nitrogen Dioxide (NO2) | Vehicle emissions, industrial sources | Photolysis under sunlight producing nitric oxide (NO) and atomic oxygen (O) | NO, O (formation of ozone) | Contributes to urban smog and ground-level ozone formation |
| Volatile Organic Compounds (VOCs) | Plants, fossil fuel combustion | Photochemical oxidation initiated by OH radicals | Formaldehyde, peroxyacyl nitrates, organic aerosols | Precursor to ozone and secondary organic aerosols, affects climate and health |
| Polycyclic Aromatic Hydrocarbons (PAHs) | Incomplete combustion of organic matter | Photodegradation by UV light leading to molecular breakdown | Smaller organic acids, CO2, CO | Reduces persistence of toxic compounds, but intermediates may be harmful |
| Chlorofluorocarbons (CFCs) | Refrigerants, aerosol propellants | Photolysis by UV light leading to chlorine radical release | Chlorine radicals (Cl*) | Depletes stratospheric ozone, increasing UV radiation at Earth's surface |
Common Atmospheric Compounds Undergoing Photodegradation
Nitrogen dioxide (NO2) readily undergoes photodegradation in the atmosphere, breaking down under sunlight into nitric oxide (NO) and atomic oxygen, which contributes to ozone formation. Volatile organic compounds (VOCs) such as formaldehyde frequently degrade via photolysis, producing radicals that drive atmospheric chemical reactions. Ozone (O3) itself absorbs ultraviolet radiation, leading to its photodegradation and influencing the oxidative capacity of the troposphere.
Role of Sunlight in Atmospheric Photodegradation
Sunlight plays a crucial role in atmospheric photodegradation by providing the energy necessary to break down pollutants such as nitrogen oxides (NOx) and volatile organic compounds (VOCs). Ultraviolet (UV) radiation initiates photolysis reactions that generate reactive radicals like hydroxyl (OH) radicals, accelerating the decomposition of harmful compounds. This process significantly influences air quality and helps reduce the concentration of smog-forming chemicals in the atmosphere.
Photodegradation of Airborne Volatile Organic Compounds (VOCs)
Photodegradation of airborne volatile organic compounds (VOCs) occurs when sunlight initiates chemical reactions that break down these pollutants into less harmful substances, significantly influencing atmospheric chemistry. Compounds like benzene, toluene, and xylene undergo photolysis under ultraviolet radiation, producing radicals that contribute to the formation of secondary pollutants such as ozone and particulate matter. This process is crucial in mitigating VOC concentrations and shaping air quality dynamics in urban and industrial environments.
Breakdown of Persistent Organic Pollutants in the Atmosphere
Photodegradation in the atmosphere plays a crucial role in the breakdown of persistent organic pollutants (POPs) such as polychlorinated biphenyls (PCBs) and polycyclic aromatic hydrocarbons (PAHs). Sunlight-driven photolytic reactions generate reactive species like hydroxyl radicals that degrade these toxic compounds into less harmful substances. This natural atmospheric process reduces the environmental persistence and bioaccumulation risks associated with POPs, aiding in pollution mitigation and ecosystem protection.
Degradation of Polycyclic Aromatic Hydrocarbons (PAHs) by Photolysis
Polycyclic Aromatic Hydrocarbons (PAHs) undergo photodegradation in the atmosphere primarily through photolysis, where ultraviolet (UV) radiation breaks chemical bonds, leading to molecular fragmentation. This process reduces the persistence and toxicity of PAHs, which are hazardous pollutants originating from incomplete combustion of fossil fuels. Photodegradation rates of PAHs are influenced by sunlight intensity, atmospheric conditions, and the presence of photosensitizers such as ozone or nitrate radicals.
Photodegradation of Nitrogen Oxides and Ozone Formation
Photodegradation of nitrogen oxides (NOx) in the atmosphere involves the breakdown of NO and NO2 molecules by sunlight, leading to the formation of reactive radicals such as hydroxyl (OH) and peroxy (RO2) radicals. These radicals play a critical role in the formation of tropospheric ozone through complex photochemical reactions involving volatile organic compounds (VOCs) and NOx. The photodegradation process influences air quality and contributes to smog formation, impacting human health and ecosystems.
Transformations of Sulfur Compounds Through Photodegradation
Sulfur compounds such as sulfur dioxide (SO2) undergo photodegradation in the atmosphere through reactions initiated by solar ultraviolet (UV) radiation, leading to the formation of sulfate aerosols. These transformations significantly influence atmospheric chemistry by contributing to acid rain and altering the radiative balance through aerosol formation. Photochemically driven oxidation of sulfur compounds plays a critical role in the sulfur cycle, affecting air quality and climate regulation.
Fate of Pesticides in the Atmosphere via Photodegradation
Pesticides released into the atmosphere undergo photodegradation when exposed to sunlight, breaking down into less harmful compounds through photochemical reactions. This process significantly reduces the persistence of pesticides such as organophosphates and pyrethroids, limiting their long-range atmospheric transport and environmental impact. Photodegradation rates depend on factors like solar radiation intensity, pesticide chemical structure, and atmospheric conditions including humidity and presence of photosensitizers.
Impact of Photodegradation on Air Quality and Climate
Photodegradation of pollutants such as nitrogen oxides (NOx) and volatile organic compounds (VOCs) in the atmosphere leads to the formation of secondary pollutants like ozone (O3), significantly impacting air quality. This process alters the chemical composition of the atmosphere, influencing the concentration of greenhouse gases and thereby affecting climate patterns. Enhanced photodegradation under increased solar radiation can accelerate smog formation and contribute to regional climate forcing.
Photodegradation's Role in Reducing Atmospheric Contaminants
Photodegradation plays a crucial role in reducing atmospheric contaminants by breaking down pollutants like volatile organic compounds (VOCs) and nitrogen oxides (NOx) when exposed to sunlight. This process transforms harmful substances into less toxic or inert compounds, thereby decreasing the concentration of smog-forming precursors and improving air quality. For example, the photodegradation of benzene and other aromatic hydrocarbons helps mitigate their impact on human health and the environment.
example of photodegradation in atmosphere Infographic
 samplerz.com
samplerz.com