Acid rain is a common environmental issue affecting mountain ecosystems, where pollutants such as sulfur dioxide and nitrogen oxides from industrial activities react with water vapor in the atmosphere. In the Appalachian Mountains, for example, acid rain has caused significant damage to soil chemistry, leading to the depletion of essential nutrients like calcium and magnesium. This process results in the acidification of streams and lakes, threatening aquatic life and impairing biodiversity in these sensitive habitats. The Rocky Mountains have also experienced the impact of acid rain, with changes observed in forest health and water quality. Trees subjected to acid rain suffer from weakened growth and increased vulnerability to pests and diseases. Scientific data indicates that acid deposition has altered nutrient cycles and reduced the availability of critical minerals, which compromises the resilience of mountain vegetation and affects overall ecosystem stability.
Table of Comparison
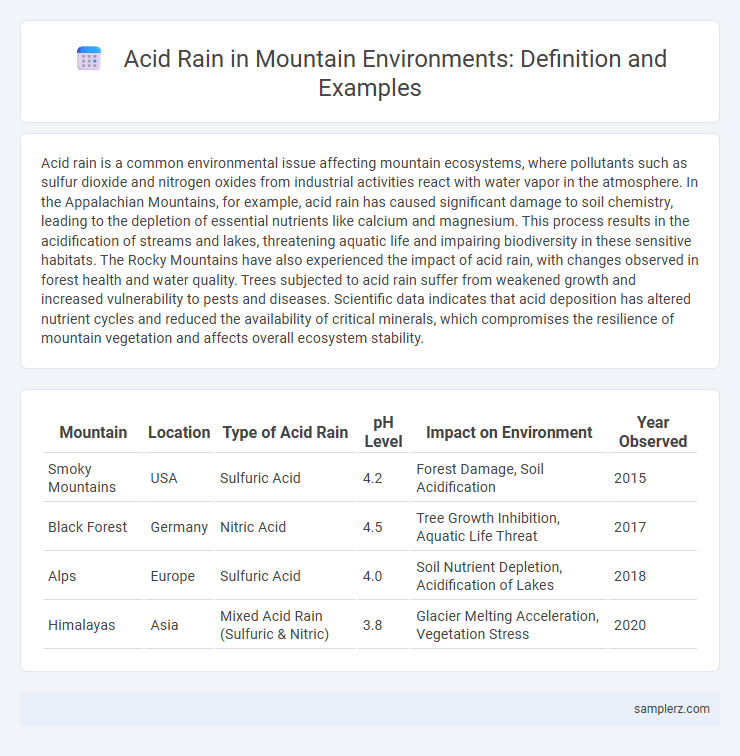
| Mountain | Location | Type of Acid Rain | pH Level | Impact on Environment | Year Observed |
|---|---|---|---|---|---|
| Smoky Mountains | USA | Sulfuric Acid | 4.2 | Forest Damage, Soil Acidification | 2015 |
| Black Forest | Germany | Nitric Acid | 4.5 | Tree Growth Inhibition, Aquatic Life Threat | 2017 |
| Alps | Europe | Sulfuric Acid | 4.0 | Soil Nutrient Depletion, Acidification of Lakes | 2018 |
| Himalayas | Asia | Mixed Acid Rain (Sulfuric & Nitric) | 3.8 | Glacier Melting Acceleration, Vegetation Stress | 2020 |
Overview of Acid Rain in Mountain Environments
Acid rain in mountain environments results from the deposition of sulfur dioxide (SO2) and nitrogen oxides (NOx) emitted by industrial activities, which react with water vapor to form sulfuric and nitric acids. High-altitude ecosystems like the Appalachian Mountains and the Rockies experience increased sensitivity due to thinner soil layers and slower decomposition rates, leading to soil acidification and the decline of native vegetation. This phenomenon disrupts aquatic habitats by lowering pH levels in mountain lakes and streams, threatening biodiversity and water quality.
Causes of Acid Rain Formation in Mountainous Regions
Acid rain in mountainous regions primarily forms due to the interaction of sulfur dioxide (SO2) and nitrogen oxides (NOx) emitted from industrial activities and vehicle exhaust with moisture in the atmosphere. The high elevation and cool temperatures in mountains enhance the absorption of these pollutants by cloud droplets, resulting in acidic precipitation. Geographic factors such as prevailing wind patterns and limited vegetation further contribute to the concentration and deposition of acid rain in these areas.
Notable Mountain Ranges Affected by Acid Rain
The Appalachian Mountains in the United States and the Black Forest in Germany are notable mountain ranges severely impacted by acid rain, leading to soil acidification and forest ecosystem damage. Elevated sulfur dioxide (SO2) and nitrogen oxides (NOx) emissions from industrial regions contribute to acidic precipitation, which accelerates the leaching of essential nutrients from the soil in these areas. Continuous monitoring and emission reduction policies have become critical to mitigate the long-term environmental degradation observed in these mountain ecosystems.
Acid Rain Impact on Mountain Ecosystems
Acid rain significantly alters mountain ecosystems by lowering the pH of soil and water, which disrupts nutrient availability and harms native vegetation such as coniferous trees and alpine plants. Studies from the Appalachian Mountains report declines in forest health and aquatic species diversity linked directly to acid deposition from industrial emissions. The resulting soil acidification reduces essential minerals like calcium, undermining tree growth and increasing vulnerability to disease and harsh weather conditions.
Case Study: Acid Rain Effects in the Appalachian Mountains
The Appalachian Mountains have experienced significant damage due to acid rain, primarily caused by sulfur dioxide (SO2) and nitrogen oxides (NOx) emissions from nearby coal-fired power plants. This acid deposition has led to the acidification of soils and freshwater systems, resulting in loss of biodiversity and forest decline. Monitoring reports highlight decreased pH levels in streams and increased aluminum toxicity, critically impacting aquatic life and forest health in the region.
Acidification of Mountain Lakes and Streams
Acid rain significantly contributes to the acidification of mountain lakes and streams, lowering pH levels and disrupting aquatic ecosystems. Mountain regions like the Adirondacks and the Rockies experience increased sulfate and nitrate deposition from industrial emissions, leading to diminished biodiversity and fish population declines. This acidification impacts water quality, nutrient cycling, and habitat suitability for sensitive species.
Forest Degradation on Mountain Slopes Due to Acid Rain
Acid rain significantly accelerates forest degradation on mountain slopes by leaching essential nutrients like calcium and magnesium from the soil, weakening tree roots and reducing overall forest health. High concentrations of sulfur dioxide (SO2) and nitrogen oxides (NOx) from industrial pollution create acid rain that damages sensitive mountain ecosystems, leading to decreased biodiversity and increased soil erosion. Studies in the Appalachian Mountains demonstrate severe declines in forest vitality linked to prolonged acid rain exposure, highlighting the urgent need for emission control policies to protect these fragile habitats.
Threats to Mountain Biodiversity from Acid Deposition
Acid rain significantly threatens mountain biodiversity by altering soil chemistry and leaching essential nutrients, which disrupts the growth of alpine vegetation. In the Appalachian Mountains, acid deposition has led to declines in sensitive species like the brook trout and various mosses, impacting entire ecosystems. These changes reduce habitat quality and increase vulnerability of mountain flora and fauna to environmental stressors.
Human Activities Contributing to Mountain Acid Rain
Industrial emissions from coal-burning power plants and vehicle exhaust release sulfur dioxide (SO2) and nitrogen oxides (NOx), which react with moisture in mountainous regions to form acid rain. Deforestation and mining activities accelerate soil erosion and reduce natural buffers, increasing mountain ecosystems' vulnerability to acidic precipitation. Agricultural practices involving nitrogen-based fertilizers further contribute nitrogen compounds, exacerbating acid rain impacts on mountain flora and fauna.
Strategies for Mitigating Acid Rain in Mountain Areas
Implementing liming techniques to neutralize acidic soils in mountain regions significantly reduces the impact of acid rain on fragile ecosystems. Reforestation with acid-tolerant tree species enhances forest resilience and biodiversity in affected mountainous areas. Strict regulation of sulfur dioxide (SO2) and nitrogen oxides (NOx) emissions from industrial sources is critical for decreasing acid rain frequency and intensity in these environments.
example of acid rain in mountain Infographic
 samplerz.com
samplerz.com