Chelation in groundwater involves the process where metal ions bind with organic molecules, forming stable compounds that affect the mobility and bioavailability of heavy metals. One clear example is the interaction between iron or lead ions and humic substances naturally present in groundwater. These chelated complexes can increase the solubility of metals, facilitating their transport through aquifers and potentially impacting water quality. Monitoring chelation processes is vital for groundwater management and pollution control. Research shows that metals like cadmium and arsenic often form chelates with dissolved organic carbon in contaminated sites. Understanding the types and concentrations of chelating agents helps in predicting contaminant behavior and developing effective remediation strategies to ensure safe drinking water sources.
Table of Comparison
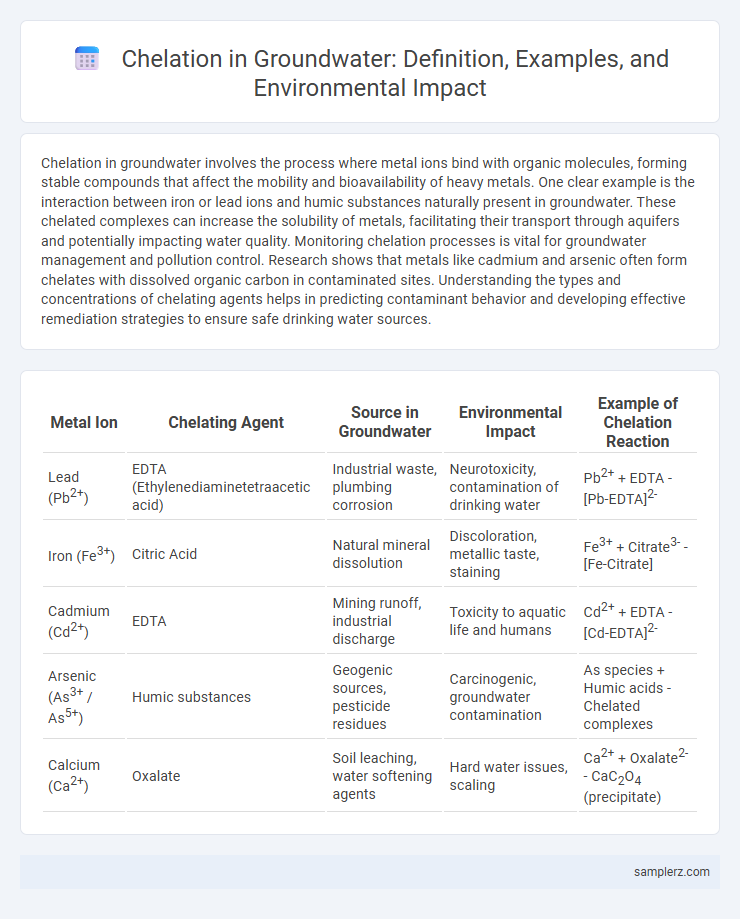
| Metal Ion | Chelating Agent | Source in Groundwater | Environmental Impact | Example of Chelation Reaction |
|---|---|---|---|---|
| Lead (Pb2+) | EDTA (Ethylenediaminetetraacetic acid) | Industrial waste, plumbing corrosion | Neurotoxicity, contamination of drinking water | Pb2+ + EDTA - [Pb-EDTA]2- |
| Iron (Fe3+) | Citric Acid | Natural mineral dissolution | Discoloration, metallic taste, staining | Fe3+ + Citrate3- - [Fe-Citrate] |
| Cadmium (Cd2+) | EDTA | Mining runoff, industrial discharge | Toxicity to aquatic life and humans | Cd2+ + EDTA - [Cd-EDTA]2- |
| Arsenic (As3+ / As5+) | Humic substances | Geogenic sources, pesticide residues | Carcinogenic, groundwater contamination | As species + Humic acids - Chelated complexes |
| Calcium (Ca2+) | Oxalate | Soil leaching, water softening agents | Hard water issues, scaling | Ca2+ + Oxalate2- - CaC2O4 (precipitate) |
Understanding Chelation in Groundwater Systems
Chelation in groundwater systems involves the binding of metal ions like lead, arsenic, and cadmium with organic ligands, forming stable complexes that influence metal mobility and bioavailability. Natural organic matter, including humic and fulvic acids, plays a crucial role in this process by altering the speciation and transport of contaminants. Understanding these chelation mechanisms is essential for predicting contaminant fate and developing effective groundwater remediation strategies.
Common Chelating Agents Found in Groundwater
Common chelating agents found in groundwater include ethylenediaminetetraacetic acid (EDTA), nitrilotriacetic acid (NTA), and natural organic acids like humic and fulvic acids. These compounds effectively bind heavy metals such as lead, cadmium, and arsenic, increasing their solubility and mobility in aquifers. The presence of these chelating agents can significantly influence metal transport and contamination risk in groundwater ecosystems.
How Chelation Affects Metal Mobility in Aquifers
Chelation in groundwater involves the complexation of metal ions such as lead, arsenic, and cadmium with organic ligands, increasing their solubility and mobility in aquifers. This process enhances the transport of heavy metals through porous media, potentially spreading contamination over larger areas. Understanding chelation dynamics is essential for predicting metal plume behavior and designing effective remediation strategies in subsurface environments.
Case Study: Natural Organic Chelators in Groundwater
Natural organic chelators such as humic and fulvic acids play a crucial role in groundwater chemistry by binding heavy metals like iron, manganese, and lead, enhancing their solubility and mobility. In a case study from the Chesapeake Bay watershed, these organic compounds facilitated the complexation and transport of trace metals, influencing both contaminant distribution and bioavailability. Understanding the dynamics of natural chelators helps in predicting the fate of pollutants and designing effective groundwater remediation strategies.
The Role of EDTA in Groundwater Pollution
EDTA (ethylenediaminetetraacetic acid) acts as a strong chelating agent that binds heavy metals such as lead, cadmium, and mercury in groundwater, increasing their mobility and persistence. This enhanced metal mobility due to EDTA complexation often leads to the spread of pollution across aquifers, complicating remediation efforts. Studies have shown that EDTA's presence in contaminated sites can worsen groundwater quality by facilitating metal transport beyond the original contamination source.
Chelation and the Remediation of Contaminated Groundwater
Chelation in groundwater remediation involves using chelating agents like EDTA to bind heavy metals such as lead, cadmium, and arsenic, enhancing their solubility and mobility for effective extraction. This process reduces the bioavailability and toxicity of contaminants, facilitating their removal through pumping or natural attenuation. Chelation techniques improve the efficiency of groundwater cleanup by stabilizing metal ions and preventing their adsorption onto soil particles.
Influences of pH on Chelation Processes in Groundwater
pH significantly influences chelation processes in groundwater by affecting metal ion solubility and ligand availability. At acidic pH levels, increased proton concentration can reduce metal-ligand complex formation, while alkaline conditions promote stable chelate formation by deprotonating ligands like humic substances. Groundwater systems with pH between 6 and 8 typically exhibit optimal chelation efficiency, enhancing the mobility and bioavailability of trace metals.
Industrial Sources of Chelating Compounds in Aquatic Environments
Industrial sources of chelating compounds in groundwater primarily include metal plating facilities, textile manufacturing plants, and pulp and paper industries, where substances like EDTA and DTPA are extensively used to bind heavy metals. These chelating agents mobilize metals such as lead, cadmium, and mercury, increasing their solubility and transport through aquatic environments, thereby exacerbating contamination risks. Monitoring and controlling chelating compound discharge is crucial for mitigating heavy metal pollution and protecting groundwater quality.
Impacts of Chelation on Drinking Water Safety
Chelation in groundwater occurs when chelating agents bind heavy metals such as lead, arsenic, and cadmium, increasing their solubility and mobility in aquifers. This process can elevate contaminant levels in drinking water sources, posing significant health risks including neurological damage and organ toxicity. Monitoring chelating compounds like EDTA is essential for assessing and mitigating groundwater contamination to ensure safe drinking water quality.
Monitoring and Detection of Chelated Metals in Groundwater
Monitoring and detection of chelated metals in groundwater rely on advanced techniques such as inductively coupled plasma mass spectrometry (ICP-MS) and high-performance liquid chromatography (HPLC) to accurately identify and quantify metal-ligand complexes. Employing sensors and probes sensitive to chelated forms of metals like iron, copper, and lead enables real-time assessment of contamination levels. Data from these methods facilitate the evaluation of metal mobility and bioavailability, critical for groundwater remediation and environmental risk management.
example of chelation in groundwater Infographic
 samplerz.com
samplerz.com